Giving thanks for both actinides and hydrocarbons
There is a pervasive myth claiming that energy is difficult to store. The objective fact is that our creator – or nature if you prefer – figured out how to store massive quantities of useable energy at the very beginning.
Aside: Refined electricity storage doesn’t appear to be difficult from a consumer’s point of view. Charging small batteries seems like such a simple task. The parts of the system that are hidden from users, however, is material intensive, consumes energy, and costs a lot of money compared to simply using electricity as soon it is generated. End Aside.
At the time of the Big Bang – the cause of which can be debated forever – a spectrum of elements formed.
Some of those elements – collectively known as actinides – were formed by pushing a large quantity of protons and neutrons tightly together. The process created atoms that were almost stable because they were held together with an almost unimaginable amount of binding energy.
Forming those actinide elements, the longest lived of which were isotopes of uranium and thorium, “consumed” a portion of the vast quantity of energy released by the Bang.
Actually, “consumed” isn’t the right word because the energy did not disappear.
For several billion years, the binding energy remained securely locked in the nuclei of nearly stable isotopes. The creator – again, nature if you prefer – gifted the key to that massive stockpile of the capacity to do work. The key was well-hidden; only creatures with curiosity, ability to document and communicate discoveries, motivation for the search, and sufficient provisions for basic survival to invest in the search would be able to discover it.
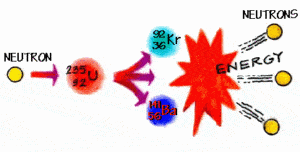
In 1932, humans found the key. James Chadwick was the person credited with taking the conclusive step in a multi-decade search when he designed and conducted a replicable experiment showing that there were particles inside nuclei that were as heavy as protons, but carried no electrical charge. Those particles, named neutrons, almost immediately became a tool of intense interest among the scientists who specialized in uncovering and describing atomic structure.
Once the key was located, it opened up an immense treasure trove whose contents are still being cataloged. We have no way of knowing how many new sections remain to be found. The trove includes rare or previously non-existent materials with unique physical properties and it included an effectively unlimited source of power to perform both constructive and destructive work.
For many good reasons – and some nefarious, selfish reasons – humans have been alternatively excited, cautious, methodical and halting during the lengthy and ongoing discovery process. We’ve learned a great deal and continue to make progress. At the same time, we’ve collectively forgotten many early lessons, ignored some useful discoveries, misused some of the knowledge, and misled even ourselves about the potential value of the information we’ve uncovered.
Fortunately, we’ve managed to avoid most potentially catastrophic results even while we have so far failed to take full advantage of the stockpile of value we’ve inherited.
It’s time to give thanks for both the gift and the progress that has been made so far in developing effective uses for the gift. It’s also time to dedicate ourselves to continued efforts to take better advantage of the gift in service to mankind.
But Don’t Forget The Value Of Stored Hydrocarbons
Nature – or our creator, if you will – provided plenty of alternative sources of power to enable the development of creatures capable of unlocking and understanding the energy stored inside atomic nuclei. Like other stars, our nearby sun used another type of power made available by the Big Bang. It was a massive enough gathering of lighter elements to enable them to be pushed together at extremely high temperatures and pressures.
When two light elements have nuclei that are mashed together, a tiny amount of their mass turns into released energy in the form of heat and a spectrum of waves ranging from low frequency thermal energy to ultra high frequency x- and gamma rays. At its distance of 93 million miles from our Earth, the portion of the Sun’s energy bathing our planet acted as a gentle stimulant for life to develop and flourish.
Some forms of life could directly convert the sun’s energy into nourishment, other forms of life developed to obtain their energy indirectly by consuming parts of the life that directly used the sun. Bits and pieces of both forms were left over and ended up being stored for future use.
There is even a reasonable theoretical probability that some forms of hydrocarbons or hydrocarbon precursors were deposited in the primordial materials that compose our home planet.
Over time, the creatures that eventually became homo sapiens learned to take advantage of chemical reactions that released heat and light from a wide variety of natural materials. They began to understand the advantages and limitations of various potential sources of combustion fuel and gradually improved the safety and utility of those forms of stored energy.
Energy stored in material gradually enabled humans to spend less time in scratching out subsistence. it enabled travel, discovery, a move away from human bondage, less dependence on animal muscle, greater comforts, improvements in heath and sanitation, better ability to store food safely and countless other components of modern living that are often taken for granted.
Even though there are some known downsides to the ways that we currently consume hydrocarbons, we should remember and give thanks for their existence and their utility. We can continue improving our methods of putting their value to work in service of mankind and we can gradually incorporate replacements in situations where those replacements provide better overall value.
It is entirely feasible to move towards a world where there is less pressure to open up new stores of hydrocarbons, but it is needlessly polarizing to talk of eliminating their use by fiat or even by propagandistic campaigns designed to use fear, guilt and demonization as tools of persuasion.
While it would be good to properly price the damage that can be done by hydrocarbon byproducts, attempting to achieve zero use is about as illogical and counterproductive as seeking to ban fire.
Aside: It’s become clear to me that coal has been demonized by people interested in seeking to capture a larger market share for methane (aka natural gas). There are benefits and disadvantages of each of those closely related fuels, but methane speculators and producers know they’ll make more money if a growing share of customers can be turned away from coal. Despite the pervasive propaganda to the contrary coal can be the best fuel choice in a given situation. End Aside.
If we are really and truly focused on quickly reducing mankind’s contribution to the ever-increasing quantity of CO2 in our atmosphere, effective action needs to include the willing participation of people that are unlikely to be convinced by fear, uncertainty and doubt. If we want major sources of capital to invest a growing share of their resources into developing and deploying fission power systems, we need them to see beneficial results from revising their investment habits.
We need to convincingly prove that fission can be even more profitable than combustion, even if the profits must be more evenly distributed to make the transition work. Though we have good reasons to be thankful for the opportunities that dense stores of energy provide, we must accept responsibility for turning potential into reality.



Actually, primordial Big Bang Nucleosynthesis pretty much ended with Lithium-7. The rest is literally the Stuff of Stars.
I like the overall article, but someone might have one technical quibble… I believe most of the heavier elements are thought to have been produced after the “big bang” by stars nearing the end of their lives as novas or supernovas. Still energy from the big bang, but somewhat indirectly.
“At the time of the Big Bang – the cause of which can be debated forever – a spectrum of elements formed.”
I normally read that the heavy atoms are created by stars going super-nova not at the time of the Big Bang.
Anything heavier than iron. Yes.
This is true. The only elements formed in significant amounts in the Big Bang are hydrogen (incl. deuterium), helium and a bit of lithium.
My understanding is that it was simply too hot for heavier nuclei to survive, and by the time things cooled sufficiently the universe was too rarefied for heavier elements to form. It took the density (and ironically, cooler temperatures) of a stellar core collapse to form actinides.
Rod – thanks for the post and the meditation on thankfulness. The quibbles can get out of hand if we forget the theme (there are actually more to be had about primordial vs. stellar generated elements). It’s only the extra power that those stored energies let us tap that gives us our modern world. Power that should be at the service of all the living world, including all humans, for benefit and not destruction.
“There is a pervasive myth claiming that energy is difficult to store.”
This is a true myth.
Many things happen in nature that we humans find difficult to do and even more difficult to do economically like storing energy. For instance, fusing small atoms to make uranium we humans find very difficult to do.
Storing energy by making hydrocarbons we have done in several ways from several different energy sources. I am most interested in making methanol from liquid fission and sea water and hope someone makes a ton of money while doing this to save to planet.
@Martin Burkle
Humans can stockpile as much energy as they could ever want by accumulating a physically modest stockpile of actinide superfuels.
That’s not particularly useful for a layperson. If I had a bar of uranium metal I could hit my neighbor over the head with it, but it would be useless to me as a form of stored energy. Not so if I instead had a chunk of coal or if I had a working watermill on my property. In this situation even a windmill would be more useful to me.
That said – how difficult is it to operate a working reactor? If you have somebody with a nuclear physics degree overseeing the reactor and assuming you have computerized the monitoring of various things where practical, is there value to having somebody that’s basically a layperson to be an extra set of eyes?
@Tavis Lucy
A barrel of crude oil would also be relatively useless for a member of the general public. That difficulty is overcome by the fact that there are companies that specialize in refining crude oil into more readily useful products like diesel fuel and gasoline – and a host of other useful feedstocks. Actinides are wonderful stores of energy; finished nuclear fuel is the form I would recommend for a stockpile at the community, factory, campus, city, state or country level.
Here’s a partial answer to your question about operating a reactor. As a 27 year old with a BS in English, I led a department of about 40 people, most just high school grads with an additional 18 months of technical training plus OJT. We operated and maintained a nuclear power plant that could have supplied reliable electricity to a small town of perhaps 15,000 – 20,000 people. That reactor was built with technology available in the late 1950s. I like to say that one of the easiest jobs in the Navy is standing watch as a reactor operator.
There are projects underway today that are striving to produce even smaller reactors that can be operated either in auto or remotely most of the time.
Stored energy, I like the idea of the Hydrogen powered car but the 5-10,000 PSI for it’s fuel tank is a bit concerning. What are your thoughts on hydrogen powered vehicles?
“Despite the pervasive propaganda to the contrary coal can be the best fuel choice in a given situation.”
How so?
The only advantage I see for coal power plants over current nuclear reactors is that they can be easily shut down, and they don’t have anywhere near the ramping ability of gas or hydroelectric.
I do recognize, though, that coal – ideally being pure carbon – is a valuable feedstock material. I just don’t know anything about coal in its various applications other than heating water to produce electricity.
For that matter, for a substance that powers countless millions of automobiles, I happen to think gasoline is relatively non-toxic. Although further research is ofcourse always a plus.
Coal can fuel direct carbon fuel cells.
There are several variants of those. Some require refined carbon (which could,
of course, come from coal) and (as I recall) one could use coal directly.
Coal has ash, and ash handling is (beyond) troublesome.
DCFCs which do not have to handle coal would be considerably easier. Given the recent advances which e.g. electrolyze molten carbonates to nanotubes and oxygen, it is likely better to avoid fossil fuels in these matters.
Various corrections have the right of it. To say more, every element more massive than iron requires a flood of neutrons. So start with a neutron star and cause it to explode by feeding it mass from a nearby companion star.
At some point we’re going to recognize that burning hydrocarbons to release energy is not the best use of these limited feedstocks that drive so many other aspects of our modern society. Using the fission of actinides to provide the energy to drive the conversion of raw hydrocarbons into the materials that drive our modern society is a much better use of these materials than simply generating heat and light.
Recent-ish thinking suggests that supernova are insufficient to produce the observed quantity of heavy elements. Recent (August 2017) observational evidence seems to confirm the neutron star collision theory.
Apparently a neutron star collision can produce Jupiter sized masses of heavy elements. I want the mining concession on those events…
I think this is the best summary I found:
https://spaceflightnow.com/2017/10/17/neutron-star-collision-an-astronomical-gold-mine/
This article contains extensive explanation of all the background information in an easy to read presentation:
https://www.quantamagazine.org/did-neutron-stars-or-supernovas-forge-the-universes-supply-of-gold-20170323/
More of a science journal type summary article:
https://www.eurekalert.org/pub_releases/2017-10/thuo-lc101617.php
Another popularization, but a shorter read than my second link:
http://www.iflscience.com/space/neutron-star-collisions-are-responsible-gold-uranium-and-other-elements/