Uranium Catalysts for the Reduction and/or Chemical Coupling of Carbon Dioxide, Carbon Monoxide, and Nitrogen
This is a guest post from NNadir, the well-known nom de plume used by a chemical engineer who has professional reasons for obscuring his off-line identity. It is far more technical than most of the posts here, but I found it fascinating. I hope most readers agree that it is a welcome respite from the energy politics and economics-related topics that have dominated posts here for the past few months.
By NNadir
Three small and seemingly simple molecules are at the forefront of the accelerating rate of the anthropogenic degradation of the planetary atmosphere. Carbon dioxide, CO2, needs no introduction. Practically every sentient being on the planet understands its importance. The profound health effects of another oxide of carbon, carbon monoxide, CO – effects including but not limited to death – are also generally well known. Although the effects of carbon monoxide as a pollutant have been to some extent ameliorated by the development of automotive (and other) catalysts, its atmospheric chemistry still remains a serious concern. What may be less well known is that an understanding of the chemistry of carbon monoxide is critical to any serious effort to address climate change, not that any serious effort will, in fact, be made. In any such effort, carbon monoxide chemistry would serve as a critical tool for humanity rather than a threat to it. The third molecule, nitrogen, N2, is the one that represents the bulk of the atmosphere, where it is generally thought of as inert, although the many exceptions to its inertness are of critical environmental, health, industrial and energetic importance that cannot be understated.
The paper from the primary scientific literature that I will focus upon in this discussion actually discusses the chemistry of all three of these molecules, each of which is enormously important to any serious environmentalist. The paper also discusses the chemistry of the “f elements,” focusing on uranium. The chemistry of the f elements is of critical importance to the environmentalist who recognizes – I include myself here – who recognizes the irrefutable fact that nuclear energy represents the most critical tool available to save humanity from itself; at least whatever portion of humanity can be saved at this late date, not that much necessarily will be saved. The long winded (at least to nonscientists) title of the paper is this: “Small Molecule Activation by Uranium Tris(aryloxides): Experimental and Computational Studies of Binding of N2, Coupling of CO, and Deoxygenation Insertion of CO2 under Ambient Conditions.”1 A link to it is found in the references.
First – excuse the digression – by allowing me to answer a question for those who don’t know: What are the “f elements?”
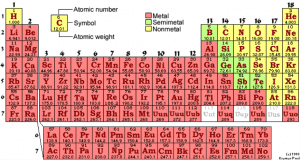
As most people who have passed a good quality chemistry course know, the shape of the periodic table (see figure 1) derives from the filling of suborbitals, the existence of which accounts for the stepwise arrangement of the table’s columns – the main orbitals are represented by each row – which are called (for historical reasons) s, p, d, and f orbitals. (Theoretically there are also g and h orbitals, but if any g and h elements have ever existed, they have done so only for transitory periods at the cores of collapsing stars.) Suborbitals are represented by columns in the periodic table: The s elements are represented by the columns headed by hydrogen (H) and lithium (Li), the p elements are headed by the columns headed by boron (B) through neon (Ne) – helium is, as always, an eccentricity and is actually an “s” element – the d orbitals are represented by the columns headed by scandium (Sc) through Zinc (Zn) and the f elements are those in the columns which are below the “main” table headed by lanthanum (La) through Ytterbium (Yb). I like the table I’ve produced here because it clearly suggests that the f elements are actually a part of the main table – another step in the staircase shape – but are usually drawn this way because otherwise the table would be generally too wide to print.
The “f elements” are distinct from the elements associated other suborbitals inasmuch as they have distinct trivial names. The elements from lanthanum to ytterbium are collectively known as the lanthanides (or sometimes as “rare earths”) and, analogously, the elements from actinium to nobelium are collectively known as the “actinides.” The lanthanides are most famously connected, in modern times, with, among other things, wind turbines, electric/hybrid cars, and certain semiconductor devices. Personally I have little use for either the wind industry or the car industry and won’t comment much further on the roles in these industries. My interest in lanthanides chiefly derives from their appearance as fission products in used nuclear fuel where those lighter than and including europium are prominently represented. (It is the accumulation a lanthanide, samarium, prominently among other elements, that is responsible for the shutdown of nuclear fuel rods before the fissionable material in them is wholly consumed. Two other lanthanides, gadolinium and dysprosium, can and do play a role in nuclear technology as neutron absorbers, although they are of trivial importance to the chemistry of used nuclear fuels.) The actinide elements (primarily thorium, uranium, and plutonium, although the chemistry of neptunium, americium, curium and perhaps protactinium and californium are relevant to any discussion of nuclear energy) represent potential or actual sources of nuclear energy. The elements beyond californium, as interesting as they may be, are unlikely to ever be of any industrial or practical significance.
This property of being “f elements” results in many similarities between the chemistry of the actinides and lanthanides, although there are important differences, most notably among the lightest actinides, in particular thorium, protactinium, uranium, neptunium, plutonium, and, to a lesser extent, americium all of which are atypical, more or less, as f element chemistry goes. The first three of the listed lighter actinides occur naturally and can be isolated from ores, two of them in vast amounts. Because their chemistry is so different from that of say lanthanum, praseodymium, and neodymium, the existence of an “actinide” series was overlooked (and was largely unimportant) until the 1940’s. Its existence was not postulated until plutonium’s discoverer, physical and inorganic chemist, Nobel Laureate, educator, diplomat, advisor to eight Presidents, and Administrator of the Atomic Energy Agency during the period of the construction of 70 of the more than 100 of the US nuclear reactors, the incomparable Glenn Seaborg, recognized it in his scientific work with the transuranium elements – many of which he discovered – thus changing the shape of the periodic table. The great development of actinide chemistry took place almost simultaneously in the 1940’s and 1950’s with the serious development of lanthanide chemistry, which up until that time was regarded as esoteric and difficult, when the development of ion exchange resins and solvent extraction techniques made the separations of both the lanthanides and actinides from one another possible on a large scale, although challenges remain to this day. Thus the f elements are among the last elements to have their chemistry explored in depth. By contrast, the chemistry of the other elements have involved centuries, and in some cases, millennia of work. Because total experience of the manipulation and utilization of these elements is relatively recent, many discoveries still remain to be made, and many already made have not been subject to full technological exploitation.
To wit, to quote the paper1 I said I’d discuss on uranium catalysts for small molecules, referring to the industrial nitrogen chemistry without which much of the world’s current population would have difficulty surviving at all the authors write:
(The bold in the quotation is mine and the superscripts therein refer to references in the original paper1.) The first bolded comment refers to the fact that the Haber-Bosch chemistry – which is essential not only to modern agricultural, but also to the pharmaceutical and other industrial chemical industries – still, nearly a century after its industrialization, involves the utilization of extreme and energy intensive chemistry. This chemistry involves the fixation of nitrogen by means of the hydrogenation of nitrogen gas to give ammonia. Everything from fertilizers, to rocket fuel, to the synthesis of practically every medication known, to many polymers, to most explosives, to the recovery and recycling of metals…the list goes on and on…depends on this chemistry.
This said, it also may be said that the boon of the “green revolution” that feeds humanity via fixed nitrogen is simultaneously a tremendous threat to humanity, not only in its environmental chemistry in surface waters where it causes eutrophication3, but also in the atmosphere, where fixed nitrogen, in particular the N2O that is an inevitable side product of the use of nitrogen fertilizers is not only the third most important climate change forcing gas, but also will soon displace CFC’s as the chief threat to the planetary ozone layer4.
Nitrogen fixation is responsible for the consumption of anywhere between 1 to 2% of the 520 exajoules of energy now consumed by humanity each year.3, 5 If this doesn’t sound like much, consider that the much ballyhooed solar and wind industries combined, despite 50 years of wild eyed cheering for them, and their consumption of vast sums of money for their “development” such as it is, have never, not once, produced as much energy in a single year as is required for just nitrogen fixation, never mind all of the other things for which we use energy. Mind you, again, almost all of the food supply on earth depends on fixed nitrogen.
Surprisingly, the formation of ammonia gas is slightly exothermic, meaning that its formation from nitrogen and hydrogen, in theory, would release energy and not consume it, but as a practical matter the synthetic path necessary – path a profound effect on thermodynamics – ultimately 32 MJ kg–1 of energy is required to fix nitrogen,3 most probably owing to the highly endothermic nature involved in the formation of the diazine intermediates which require the breaking of a highly stable nitrogen-nitrogen triple bond.6 Thus, again, the industrial synthesis of ammonia in quantities exceeding 100 million metric tons – a rough estimate of the amount synthesized each year – requires exajoule scale quantities of energy.
So in terms of energy efficiency, a route that would form ammonia via less harsh conditions than those of the Haber-Bosch system and this is why the uranyl complexes described in reference 1 are so interesting. The nitrogen complex is surprisingly stable, and its structure suggests that it may well represent a route around the diazine intermediate, greatly reducing the energy requirements for nitrogen fixation. In some ways, the use of uranium for this type of chemistry should be unsurprising, since Fritz Haber, the fascinating scientist – I highly recommend for further reading Smil’s book referred to in reference/note 3 – who won the Nobel Prize for his work on nitrogen fixation wrote in 1909, around the time of his discovery, the following text7:
This translates as follows:8
It seems that humanity forgets important things. (Although Haber was a brilliant physical chemist as well as a brilliant inorganic chemist – he claimed to have discovered the third law of thermodynamics before Nerst – there is no way that he understood the theoretical basis for uranium catalysts: Recall that he understood uranium as a d-element, a heavier analog of tungsten.)
Let me now turn to the other two other small molecules in which uranium offers catalytic possibilities: Carbon dioxide and carbon monoxide.
I am not fond of dangerous fossil fuels and believe they should all be phased out on an emergency basis, even as I recognize that this is not easy to do, although I expect it would be less troublesome, over the long term, were such a phase out to occur (and I don’t believe it will do so except by catastrophe) than the more likely alternative of doing nothing meaningful. Nor am I fond of one hopelessly inadequate proposed response to the catastrophe of carbon dioxide accumulations, generally referred to as CCS, carbon capture and storage, which is a proposal to build massive but probably leaky and temporary waste dumps all over the planet to try to put said carbon dioxide out of mind. A new marketing term for “CCS” has appeared – and actually it makes more sense – which is called “CCSU,” “Carbon Dioxide Capture, Storage and Utilization.” If you must know, I approve, maybe, of what the “U” stands for and maybe, under more limited conditions what the second “C” stands for. I have trouble with the “S” though. I oppose, in general, waste dumps of any kind.
In theory, this uranium catalyst could be useful for the utilization of carbon dioxide, although many other catalysts that can do the same thing are widely known. For instance, references 7 and 8 are the same description in two languages of a process to prepare a methanol derivative from carbon dioxide via hydrogenation using a uranium catalyst. Methanol potentially represents a fuel to displace gasoline and other motor fuels.9 If the carbon dioxide source is a dangerous fossil waste stream, the hydrogenation could represent a carbon neutral path to motor fuels – allowing the phase out of petroleum and petroleum mining – or better yet, in the case of carbon capture from the air, possibly via a biological intermediate or even by direct capture, then motor fuels might actually represent, briefly at least, a carbon negative technology.
The preparation of methanol from carbon dioxide requires the breaking of one carbon oxygen bond, not actually an easy trick, although many technologies for doing so are well known.
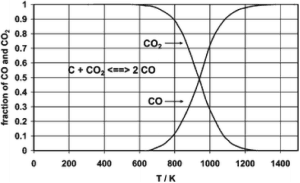
One my personal favorites involves the oxidation of a carbon source – this is known as an equilibrium associated with what is called the Boudouard reaction – with carbon dioxide itself to give carbon monoxide; this works not only with dangerous fossil fuels, but also with things like biomass: The appearance of soot on smokestacks is actually the result of a Boudouard equilibrium, running in the reverse of the previously stated route: The position of the equilibrium is temperature driven, and under some conditions carbon monoxide can disproportionate into carbon and carbon dioxide. (See figure 2.)
An examination of Figure 2 however points to a drawback associated with the reduction of carbon dioxide with either carbon or a carbon surrogate such as cellulose, waste plastic or other such substance: The drawback is the requirement for heat, which is, of course, a form of energy. In the (somewhat simplified) graph shown, the equilibrium point at which an equimolar quantity of the monoxide and dioxide is present, is a little less than 1000K, approximately 700oC.
Although modern materials science makes these kinds of temperatures accessible without access to dangerous fossil fuels – the best option being nuclear energy, albeit using reactors of types not routinely deployed – it would be convenient for some purposes to carry out such a reaction at lower temperatures and generally milder conditions. Again, it is, in general, difficult to break a carbon-oxygen bond in carbon dioxide, but obviously not impossible, since chlorophyll accomplishes this photochemically indirectly via photochemical water splitting and de facto hydrogenation. (It is also possible to hydrogenate carbon dioxide in chemical reactors).
A very convenient means of carrying out this reaction would be electrochemically. Some progress in this area has been gathering attention, for instance, in the work of Andrew Bocarsly at Princeton University and his startup company Liquid Light, apparently – if I have this right – by means of the electrochemical transfer of a single electron from an organic pyridinium radical to carbon dioxide11 via a radical carbamate intermediate.12 (The single electron reduction of linear carbon dioxide to form the bent CO2●- radical is the most energetically expensive step in the reduction of carbon dioxide to hydrocarbons, alcohols, or for that matter, in biological settings, sugars.6) The product of the Bocarsly reduction of carbon dioxide is methanol.
With all due respect to this approach which is elegant in its simplicity, it is of some note that the Arnold uranium moieties described in reference 1 directly breaks carbon oxygen bonds as well by, apparently, a different mechanism, followed by the insertion of a CO moiety in a phenolic oxygen-aryl bond (in ligands coordinating to uranium) resulting in an aryl carbonate and does so under fairly mild conditions. Carbonates are very useful in a wide variety of situations, including but hardly limited to use in the formulation of motor fuels that burn considerably cleaner than other fuels. This is an oxidation/reduction reaction, where the reduced species is carbon dioxide, The oxygen from the broken bond is inserted as a bridge between two uranium atoms.
One important difference between other catalysts is that the Arnold and other uranium catalysts may exhibit a particular type of selectivity, to make what we call C2 carbon compounds. The most important, industrially, C2 is probably ethene, colloquially known as “ethylene,” which is a either key intermediate or potential key intermediate in the production of polyethylene, acetic acid, acrylic acid (a C3 compound accessible from C2 starting materials), ethanol (without the requirement for agricultural land), ethylene glycol, ethylene oxide and a bunch of other chemicals and materials that are critical to modern bourgeois first world life even if we don’t appreciate as much.
This consideration arises uranium catalyst described in the paper actually results in the coupling of two carbon monoxide molecules – we call this dimerization – to form a type of structure we call an “ynediolate ethers” which is an highly unsaturated analogue of better known (and industrially important) vinyl ethers. Ynediolate ethers are very difficult to make even under laboratory conditions; very few are known. This is noted in the text of the paper, and although the paper does not examine them in detail as catalysts to make these molecules, instead focusing on more esoteric structural and bonding issues surrounding the uranium complexes themselves, one can easily imagine all kinds of interesting applications for this sort of molecule. One application that suggests itself is the preparation of light weight organic conducting polymers, for instance, the preparation of which might sequester a little carbon on the side. (For now, mostly they are made to make compounds like organometallic complexes of esoteric interest, such as squarate ethers; there are several varieties of uranium catalysts suitable for squarate synthesis these are of potential interest to medicinal chemists for the preparation of certain classes of cancer compounds known as tyrosine phosphatase inhibitors.)
A somewhat broader, if brief, discussion of the activation of small atmospheric molecules using actinide complexes is available for further reading.14
I am not sure whether any of this will prove to be of practical interest, although it is quite possible that at least for the potential of nitrogen fixing catalysts that require less energy might be developed. I discussed this topic with my fourteen year old son as I was writing this piece – I have raised him to be as much of a pronuclear environmentalist as I am, and he has already developed enough cynicism to state that people might object to their food being grown by any means which involves uranium. I laughed and told him about something of which he was unaware, that uranium and fertilizer have been long involved with one another. It may be true that fixed nitrogen can be regarded as an inexhaustible resource so long as there is energy to fix it, but another critical component of fertilizer is, of course, phosphate which is mined from deposits around the world and, being somewhat more problematic to recycle, might well face depletion in the future. Uranium, it turns out, has a high affinity for phosphate, so much so that for many years, many phosphate resources have been recognized as potential uranium ores. In fact, in the 1950’s, plants15 to do precisely that operated, until uranium resources became more widely known and cheaper ores were found at the same time that interest in nuclear power waned under an assault of fear and ignorance. As a result the uranium was just left in the phosphates, where, in many cases it ended up being distributed on agricultural fields. The issue of uranium accumulations in agricultural fields from phosphate fertilizers is now recognized as a worldwide health issue. 16, 17, 18
Recently it has been discovered that thorium complexes can provide a route to the ynediolate ethers19 mentioned above, much like uranium complexes, suggesting that in many ways, as one would expect, the actinides behavior similarly owing to the availability of f orbitals. A recent survey of the non-aqueous complex ion chemistry of the actinides20 notes that there are fewer entries in the Chemical Structural Database between 2006 for the organometallic complexes of all the actinides (there are 4164 such entries, with more than 84% of them being uranium complexes) are easily outstripped by the number for those of the single elements copper (41668 entries) and iron (33303 entries.) There are only 77 entries for plutonium, 134 for neptunium, 17 for the elements americium, curium, berkelium and californium combined. Given little gems like those described above to perform chemistry on molecules of profound and urgent importance to humanity, I personally regard this as a disgrace. Of course, all of these elements are radioactive, and we know merely from how the word “radioactive” is used in our lexicon how much fear, ignorance, superstition and mysticism is invested in the word. The chemistry and nature of the 5f orbitals of actinides are poorly understood, and much confusion about their exact properties – mostly obtained by theoretical modeling – exists. It seems almost certain however, that many important and potentially very useful properties have been overlooked. The synthetic elements neptunium (the 237 isotope) and plutonium (isotopes 242 and 244) have isotopes that are sufficiently long lived to be easily managed in catalytic systems with lower risk, to be sure, than the potentially greater risk associated with forswearing a deeper understanding of them. This especially applies to the wonderful element plutonium, which besides being a fabulous source of energy is a kind of chemical chameleon. (Conceivably one could make curium, 147Cm, with low enough radioactivity to be easily handled as a catalyst, but it is difficult to imagine any such property that would be unique enough justify the expense of doing so.) There are various routes to relatively pure samples of these less radioactive actinide isotopes, via prolonged neutron irradiation of lower actinides or even higher actinides. (The higher plutonium isotopes, 242Pu and 244Pu – and one important lower isotope 238Pu – may be formed by prolonged neutron irradiation of americium for example, with the isotopic purity being a function of the precise preparation procedures used.)
In recent months, a widely read paper in the scientific literature22 has conclusively demonstrated what should have been obvious long ago, that nuclear energy saves lives. Nevertheless, fear and ignorance, to beat a horse that is not dead and still needs beating, has prevented nuclear power from doing all that it can or might have done. Indeed, as I hope this discussion suggests, it may be that the materials in used nuclear fuels might be better understood as potential keys to the future – and not just as fuels – and not as the horrible objects of terror that have been presented as by some of the smaller, if popular, minds who have discussed them bereft of any understanding of what they are and what they actually can do.
References and Notes
1. Polly Arnold, Nikolas Kaltsoyannis and Steven Mansell. J. Am. Chem. Soc. 2011, 133, 9036–9051.
2. http://www.chemglobe.org/ptoe/ (Accessed July 4, 2013.)
3. Jan Willem Erisman, Mark A. Sutton, James Galloway, Zbigniew Klimont and Wilfried Winiwarter, Nature Geoscience 1, 636 – 639 (2008) One reference in this paper is to the fascinating Enriching the Earth: Fritz Haber, Carl Bosch and the Transformation of World Food Production (MIT Press, Cambridge, Massachusetts, 2001) by the always interesting Vaclav Smil. It’s an excellent historical view of how humanity and much of the other forms of life became dependent on industrially fixed nitrogen in less than a century.
4. A. R. Ravishankara, John S. Daniel, Robert W. Portmann Science 326, 123-125 (2009)
5. Richard Schrock, PNAS 2006 103 (46) 17087
6. James B. Howard and Douglas C. Rees Chem. Rev. 1996, 96, 2965-2982
7. Oanh P. Lam und Karsten Meyer Angew. Chem. 2011, 123, 9715 – 9717
8. Oanh P. Lam and Karsten Meyer Angew. Chem. Int. Ed. 2011, 50, 9542 – 9544
9. George A. Olah *, G. K. Surya Prakash , and Alain Goeppert J. Am. Chem. Soc. 2011, 133, 12881–12898. George Olah, a chemistry Nobel Laureate now in his eighties, has been a tireless worker for closed carbon cycles, most of which are based on DME (dimethyl ether) or methanol. He has published a large number of papers published on this topic, but the cited paper is a nice review article.
10. Reinhold Kneer, Dobrin Toporov, Malte Förster, Dominik Christ, Christoph Broeckmann, Ewald Pfaff, Markus Zwick, Stefan Engels and Michael Modigell, Energy Environ. Sci., 2010, 3, 198-207 Modigel
11. Emily Barton Cole, Prasad S. Lakkaraju,† David M. Rampulla, Amanda J. Morris, Esta Abelev, and Andrew B. Bocarsly,. J. AM. CHEM. SOC. 2010, 132, 11539–11551
12. Amanda J. Morris, Robert T. McGibbon, and Andrew B. Bocarsly ChemSusChem 2011, 4, 191–196
13. Daniel L. DuBois, Stephen W. Ragsdale, Thomas B. Rauchfuss et al. Chemical Reviews, ASAP Online, Accessed July 3, 2013.
14. Polly L. Arnold, Chem. Commun., 2011,47, 9005-9010
15. J. Agric. Food Chem., 1953, 1 (4), pp 292–292
16. N. Yamaguchi⁎, A. Kawasaki, I. Iiyama, Sci.Tot.Environ.407, 1383–1390
17. Hubert Tunney, Mirjana Stojanovic´ , Jelena Mrdakovic´ Popic´, David McGrath, and Chaosheng Zhang J. Plant Nutr. Soil Sci. 2009, 172, 346–352
18. R.A. Zielinski, K.R. Simmonsa, W.H. Orem Applied Geochemistry 15 (2000) 369-383
19. Huidong Li, Hao Feng, Weiguo Sun, R. Bruce King, and Henry F. Schaefer, Inorg. Chem. 2013, 52, 6893−6904
20. Matthew B. Jones and Andrew J. Gaunt, Chem. Rev. 2013, 113, 1137−1198
21. Nikolas Kaltsoyannis Inorg. Chem. 2013, 52, 3407−3413
22. Pushker A. Kharecha* and James E. Hansen Environ. Sci. Technol. 2013, 47, 4889−4895



Glad to see you’re still alive and kicking. Like many, was shocked by the boot from DKos, but hope to see you posting here (and elsewhere) in the future.
He was booted from DKos? Wow, I bet they’d toss me in a microsecond if I showed my face again.
Well, Keith, it was a long time coming.
Daily Kos always was, is, and always will be an anti-nuke website. Kos is an anti-nuke as is evinced that all of the front pagers he has promoted are anti-nukes. (My personal favorite among the front pagers has to have been that woman – she no longer writes there apparently – who liked to represent the fact that her summer internship job at Hanford made her qualified to rule on the value of Glenn Seaborg’s work.)
As a Democrat, and as an environmentalist, I am both embarrassed and appalled by the anti-nuke wing of my political party, and although I always knew that the majority of Kossacks (as opposed to the general class of Democrats) were anti-nukes. To Kos’s credit, as an anti-nuke, he gave me a wide berth to express my opinions, even though I was always deliberately skating at the edge. As a result I had the pleasure of receiving occasional notes and comments from people who wrote me to tell me I had helped them change their minds about nuclear energy. But frankly, we were at the point of diminishing returns.
I stopped writing diaries at Daily Kos – for the second time – in December of last year. The destruction I observed in my home state, New Jersey from Hurricane Sandy, as well as the extreme temperatures and droughts that have struck here and elsewhere in recent years, the deaths of ancient trees, and above all the continuous monitoring of the data from the Mauna Loa carbon dioxide observatory, particularly the effects of the irrational post-Fukushima panic that can be discerned from that data, have all conspired to raise my disgust with anti-nukism to levels that were very difficult to contain. Thus whatever comments I made (outside of my jokes on SensibleShoes wonderful Write On! Series of diaries) were seething with a sense of horror at what anti-nukism has wrought. I was especially angered by the disingenuous and frankly fraudulent remarks made by that old tiresome anti-nuke fool Tim Lange (Meteor Blades) which was of the “I’m not an anti-nuke” anti-nukism whereupon he listed all of the insipid anti-nuke arguments one after another, although not one of them despite their fifty year age would stand a fifty second scrutiny in comparison to all other forms of energy.
I take great pleasure in the fact that the thing I was banned for was for telling what I regard as the absolute truth. It actually happened in your diary. It happened in your diary where I pointed to Hansen’s recent – and increasingly famous – paper in Environmental Science and Technology wherein he quantified degree to which nuclear energy saves lives. Pointing to it, I said that it followed from the paper that anti-nukes were guilty of murder and that the murder weapon was fear and ignorance. I used that word, murder. Maybe I should have used a wimpy word like, say, um, “manslaughter” but that’s not my style and I was – and am – in fact fed up. (I hate being accused of ambiguity.)
Kos’s rule is that people should treat his website – his personal profit making website – as if it were someone’s home. Think about it. Kos is an anti-nuke. All of his prominent guests in his “house” are anti-nukes. Someone walks into your “house” and announces that all of the guests are in a class that can only be described as “murderers.” What would you do? We cannot fault Kos if his reaction is to shoot the messenger, can we?
Of course, if someone believes that the members a group he hangs out with are, in fact, murderers, then it would be a pointed and difficult question to ask what, exactly, one is doing hanging out with said group.
As Hansen has pointed out, “Nuclear energy saves lives, not every life – and sometimes, albeit rarely it costs particular lives – but on balance it saves lives.
Now.
Let’s erase nuclear energy from this equation and talk about heart surgery, which overall, saves lives. Suppose we meet a child who has “blue baby” syndrome, an often fatal condition that can only be treated surgically. Suppose also that the baby has totally naïve parents who knows little or nothing of the world. Next we have a group of people who point out all of the people who have died during heart surgery, ignoring all of the people who were saved by heart surgery. They announce, based on this selective listing, that heart surgery is dangerous, and that everyone who has heart surgery will eventually die (ignoring the fact that people who don’t have heart surgery will all die) as well. If a parent is convinced by these absurd arguments not to have the baby operated on, and the baby dies from the lack of treatment, might we not argue that the people who convinced the parents to do the wrong thing have responsibility for causing the death of the baby. What is the word for causing the death of another person?
My political views are informed by an Eleanor Rooseveltian philosophy which mixes in things like concern for the poor, the hope to provide all humans a chance to lead decent and productive lives, the creation of opportunity, a demand for justice, a desire to work forcefully for peace. Many of the great pioneers of nuclear energy were Democrats, liberal thinkers, and the greatest as far as the construction of the American nuclear infrastructure (now under assault) Glenn Seaborg. Seaborg saw the problems rising in our party – his remarks on Michael Dukakis in his book A Chemist in the Whitehouse speaks volumes. The point of electing Democrats should not be to defeat Republicans – which seems to be Kos’s ideas – but to provide a basis for good government.
Is the restriction of nuclear energy because it does not meet arbitrary criteria that no other form of energy can meet as well good government?
Kos and his front pagers and I have fundamental differences that cannot be bridged. I assure you that it is no loss to him, and at this point, it’s no loss to me either. I did what I could, but it could never be enough. I wish I could tell you that nuclear energy will triumph, but that’s not what I really believe. What I believe is that – as was often the case at so many other points in history – fear and ignorance will triumph.
Unfortunately one must choose from one of two parties and have few tools to address this crisis politically. I have always chosen to vote Democratic, and I can’t imagine that I could ever vote Republican, but my experience at Kos has caused me to reflect that there should be some times where one supports neither party. I voted for Dukakis, holding my nose. But I can tell you that I was very happy to not live in Massachusetts, because rather than vote for Markey, quintessential anti-nuke, I would have stayed home, because nuclear energy saves lives and it follows therefore that if one opposes nuclear energy then, well, I’ll say it, one is guilty of murder.
On a personal level, thank you for your kind words.
The conclusion of my Kos tenure is no great loss for me personally. In the end it only made me apoplectic.
Many months back, as I contemplated my inevitable banning from Kos, my friend Brian Mays, a fine nuclear engineer and scholar, did me a huge favor and downloaded all of my Kos diaries and emailed them to me, so whatever personal value they have for me and my sons when I am gone will not be lost.
Rod is also a friend, and a tireless worker for nuclear energy, and perhaps he will indulge me to write here again when the mood suits either or both of us. I do kick around on the internet here and there and please be assured that I continue my reading and my thinking about nuclear energy. You may contact me about any questions you may have with which I may be able to help at my email account NNadir.fp@gmail.com.
All this said, I am profoundly disturbed by the contempt for science – genetic as well as nuclear science – on the left and think it to be equally as odious as the contempt for science on the left. These things threaten not only our country, the futures of our children and grandchildren but also our species, our entire planet, as well. As I contemplate what’s going on in the country of Seaborg, of Gibbs, the country where so many of the great nuclear pioneers came to work freely, I am increasingly filled with a sense of hopelessness.
Re: “…Pointing to it, I said that it followed from the paper that anti-nukes were guilty of murder and that the murder weapon was fear and ignorance. I used that word, murder. Maybe I should have used a wimpy word like, say, um, “manslaughter” but that’s not my style and I was – and am – in fact fed up. (I hate being accused of ambiguity.)”
No, it’s not hyperbole stating that! They’re mass murderers by the consequence of their anti-nuclear deeds. We should never pussyfooting PC around when comes to many millions of lives lost daily to bad water and no energy denied because of the off-the-wall nightmares and philosophical anti-nuke hangups of a couple of groups who want to save the world like green crusaders for Gaea-approved wind and sun power. I have relatives and friends in Africa who tell how early on Greens flew in from outside to persuade all the ignorant susceptible natives not to spoil their paradise home of humankind by stroking the evil god nuclear — Oh yes, also don’t mow savannahs and jungles over with windmills and solar farms because mankind’s homeland must be kept pristine to visit! — before flying off like they did another grand deed for mankind, but nary a wilt of these “earth do-gooders” — who hawk that best way to cure malaria in Africa is for citizens is to sleep under free nets — never stop by the back country to see all the wasting and thirst and starvation and withered crops that could’ve been avoided by clean low-footprint nuclear power and generated water. Freaking hypocrites and a half! Damn them all to a one! If only these anti-nuke con artists’ legions of clueless fretful fans could see a Nat Geo on how many real life — not speculated stats and figures of fanciful nuke perils, but REAL lives that their anti-nuke heroes are killing off every week by frustrating and denying others the use of proven clean safe power and water! Thank god some of these governments have members not so gullible and are obliging Russian offers to build reactors by. So yes NNadir, call the beasts and bastards by what they ARE by their their selfish egotistical irresponsible actions. Murders of millions every year! I’m only sorry Pandora didn’t go the route of showing the brutal real life and death en masse consequences of FUD and ignorance.
James Greenidge
Queens NY
I like your comments and articles. Concerning creeping hopelessness, I believe that we should do what we do because we have determined that it is right, but we should suppress our attachment to the fruits of what we do. In other words, we should not be overly happy when the fruits are sweet, and we should not be overly dismayed when the fruits are bitter. Because that’s just life. Nevertheless, I fully understand the feeling of hopelessness that you describe. For what it’s worth, I’m now just contributing something that I learned from a brief contact that I had years ago with the ancient Hindu scriptures, which contain a lot of guidance on how important it is for one’s happiness to simply do one’s duty with dedication, but without attachment to the results.
Best regards, and I hope to read more of you. I aim to read your existing articles as well.
Joris
Re: “…Pointing to it, I said that it followed from the paper that anti-nukes were guilty of murder and that the murder weapon was fear and ignorance. I used that word, murder. Maybe I should have used a wimpy word like, say, um, “manslaughter” but that’s not my style and I was – and am – in fact fed up. (I hate being accused of ambiguity.)”
No, it’s not hyperbole stating that! Anti-nukers ARE mass murderers by the horrific consequence of their anti-nuclear actions. We should never PC pussyfooti around when comes to many millions of lives lost yearly to bad water and no energy being denied them because of the nuclear plant-blocking off-the-wall nightmares and philosophical anti-nuke hangups of a couple of groups who want to save the world like green crusaders for Gaea-approved wind and sun power. I have relatives and friends in Africa who tell how early on Greens flew in from outside to persuade “all the ignorant susceptible natives not to spoil their paradise home of humankind by stroking the evil god nuclear –” (my non-PC “sic” as a black American laying on that infamous African missionary tome!) “– Oh yes, also don’t mow savannahs and jungles over with windmills and solar farms because mankind’s homeland must be kept pristine to visit!” — before smugly flying off like they did another grand deed for mankind, but nary a wilt of these “earth do-gooders” — who hawk that the best way to cure malaria in Africa is for citizens is to sleep under free nets(!) — never stop by the back country to see all the wasting and thirst and starvation and withered crops that could’ve been avoided by clean low-footprint nuclear power and generated water. Humane hypocrites and a half and damn them all to a one, excuse my French! If only the clueless fretful fans of these anti-nuke con artists could see a Nat Geo on how many real life — not speculated stats and figures of fanciful nuke perils, but REAL lives that their anti-nuke heroes are killing off every week by frustrating and denying others the use of proven clean safe power and water! Thank god some of these governments have members not so gullible and are obliging Russian offers to build reactors by.
So yes NNadir, call the beasts and bastards by what they ARE by their selfish egotistical irresponsible actions. Defacto murderers of millions every year who go unrecorded in the media! I’m only sorry Pandora didn’t go the route of showing the brutal real life and death en masse consequences of FUD and ignorance.
James Greenidge
Queens NY
NNadir – It was a pleasure, and it was not much trouble at all. In a former life, I was a computer geek, who was involved in the Free Software movement (which is something I still support, by the way … and yes … I’m still a computer geek).
More importantly, however, it should be clear to anyone who has read your writings that you have put great care into your work. (Even those who disagree with your position have acknowledged that.) It would have been a crime if you had not had your own personal copy of these essays.
I enjoy your posts NNadir. However, on the subject of “murderers”, I suspect that stepping back from stridency can often be more effective. In regard to the liberal, faux green anti-nuclear zealots, I prefer to think of them, and refer to them, as “useful idiots” of the fossil fuel lobby. Pathetic, minor accomplices to the horrible death toll of the coal industry might actually be more accurate. Also, on the subject of pessimism, I would think that a chemist such as yourself would be familiar with F. Engels “Dialectics of Nature” where he demonstrates that all motion involves the transformation of small, quantitative changes into large, abrupt qualitative changes, and vice versa. This is easily demonstrated in chemistry. Colloquially, “tipping points” also exist in historical motion, and are usually, very surprising and unexpected. We should not project some of today’s distressing trends in re. to nuclear energy, too far into the future, in my opinion.
“We should not project some of today’s distressing trends in re. to nuclear energy, too far into the future, in my opinion.”
I agree, but I guess pro-nuke advocate’s have been telling themselves that for 30 years? Still this time it may be different. I do see things are changing. 30 years too late, but they are changing.
The anti-nuke argument that goes “it is too late for nuclear to play a role in mitigate global warming” is the one that makes my blood boil. After all, who but the anti-nukes are responsible for nuclear being ‘too late’ to begin with? These people seem to have no shame….
BTW, I like the term “useful idiots”. I had a correspondence with a dutch group that presents itself as an authority on green building in order to move them to support SMR’s as a legitimate way to decarbonise the built environment. When that group finally decided to dismiss my motion even while admitting they had no arguments against it other than a ‘feeling’ that nuclear was not sustainable, I accused them of ‘undermining society’.
Rod – thanks, as always, for this post. I’ve read all of nNadir’s posts at DailyKos and they’ve been important to my nuclear education.
nNadir – thank you for this informative post. I remember enough of my first year university chemistry to be able to follow it in general. I had been wondering if there might be ways to do something with CO2 if we were to start removing it from the air and oceans. Ideally we’d turn it into something useful, rather than just stabilizing and burying it; I’d like to see some of it turned into graphite, preferably pure enough to be nuclear grade. (Dr. Bruce Hoglund has interesting things to say about graphite in molten salt reactors in his presentation Bon-Bon Road to Core Wall Neutron Flux Suppression @ TEAC5. The conference videos are being posted gradually; I recommend watching them all.)
It’s also interesting that Helen Caldicott and other antis have missed yet another nuclear catastrophe:
What’s ironic is that they’re chasing the will-‘o-the-wisp of radiation from nuclear reactors and ignoring a real source of exposure. A quick Google search on ‘uranium in fertilizer’ turned up over a million links, and a quick skim of some of them shows the major concern is radiological. I’d have thought someone would have included heavy metal toxicity in the mix.
I have a long piece written on the subject of carbon dioxide capture from the air (and its relationship to nuclear energy). If Rod agrees, I may publish it here.
It’s a very challenging topic.
There appear to be a number of very simple and cheap methods, such as the potassium carbonate absorber system. All that one requires is heat to regenerate the absorbent, at 125°C or thereabouts. Steam tapped off from the low-pressure turbines of nuclear plants would be perfect for this; it could be used to modulate power production during off-peak hours.
The real problem is finding the energy to do something with the CO2.
@Engineer-Poet
I think you vastly overestimate the efficiency of absorber systems and underestimate the quantity of heat required. My experience is that even the best of absorbers begin to have difficulty bringing CO2 concentration much below 0.5% – 1%. (Please remember, 1% is 10,000 ppm.)
A better way seems to be to remove the carbon from sea-water, like that recent research by the US Navy appears to have confirmed. As I recall, that research was performed to investigate the feasibility of making synthetic jet fuel on board nuclear navy ships. I also recall that the energy/economic cost of obtaining the needed carbon from the seawater was a relatively minor part of the total cost of producing the synfuel. That seems to indicate that removing carbon from seawater could be an efficient way way to remove carbon from the carbon cycle. Conversely, getting carbon directly from the smokestacks of fossil power plants is even easier, but I recall that you can only reasonably get about 90% of the carbon removed in that way. That’s a lot of carbon, but not quite enough to obtain the much-hyped ‘clean coal’ situation. The carbon intensity of elektricity generation should be less than 50 grams/kWh if global warming is actually to be stopped. Current proposals for carbon capture and storage for coal don’t achieve that level of co2 emissions reduction, AFAIK.
In any case, AFAIK carbon capture and storage may be technically feasible and economically bearable, it will never be applied on a large enough scale without a significant carbon tax in place globally, which would seem unlikely to happen any time soon.
Rod wrote:
They’d need to be a lot better than that to be usable for spacecraft life-support, wouldn’t they? IIUC people don’t tolerate 1% CO2 for very long.
K2CO3 was proposed as the absorbent in the “Green Freedom” scheme, grabbing CO2 from the stream of air passing through the system’s cooling towers. I’m not a chemist, so if they got it wrong, I cheerfully plead lack of responsibility for their numbers. If anyone has firm info, I’d love to have it (for bookmarking and inclusion in the References section on The Ergosphere, if I ever get back to it).
Joris wrote:
I checked out the numbers from the lab test, and the ELECTRICAL input came to about 1 MJ per mol of CO2 produced. That’s roughly equal to the energy of the required hydrogen to produce longer-chain alkanes. I wouldn’t call that a “minor part”, especially given the 2/3 losses in conversion from heat.
I am not a fan of biofuels nor CO2 sequestration, but there one use for both together — biofuel electric generating plants with CO2 sequestration for the purpose of removing CO2 from the atmosphere.
We should not be using biofuels for electric generation (except perhaps peaking). If we are going to use biofuels, we should reserve them for high-value purposes like liquid fuels and chemical feedstocks. If we make them with nuclear process heat and capture any byproduct CO2, then the system as a whole can easily be carbon-negative.
NNadir – I also would like to read your piece on carbon dioxide capture from the air. There’s a company here in Calgary, Carbon Engineering, who are attempting to commercialize a hydroxide to carbonate to regenerated hydroxide cycle for removing CO2 from air. Their January 7, 2013 post Outdoor Contactor Data Analysis shows charts and discusses the results of their summer/fall 2012 contactor run.
Jim Holm at his continuously changing Coal2Nuclear or Skyscrubber site, is trumpeting CO2 capture as complementary to replacing coal fired boilers with high temperature nuclear reactors. He visualizes uses the reactor’s heat at night to regenerate the hydroxide and turn the CO2 into hydrocarbon fuels, and during the day to generate electricity. I’m a little skeptical of this – it seems to me that industrial scale chemical processes don’t like being ramped up and down too often. But I admire Jim’s vision, and replacing coal boilers with reactors would certainly conserve assets.
On the ‘murder’ topic – a couple of years back I wondered how much of a murderer I was by using coal-fired electricity (a good bit of our power here in Alberta comes from coal). Depending on the numbers you use for annual coal related deaths, I figured that over my lifetime I am/will be responsible for about 0.2 of a death. It’s not as bad as I feared but is still a lot higher than it should be. (I posted this idea in a comment on NNadir’s blog some time back.)
Do not despair – Ben Heard at DecarboniseSA is showing us that progress is possible. His latest post is Strong signs of progress in opening Australia’s nuclear conversation. It can be done.
It was a long time coming.
Daily Kos was, is and always will be an anti-nuclear website, it represents that unfortunate and embarrassing wing of my party, the Democratic party represented by its anti-nuke wing, the Dukkassites, if you will. (Glenn Seaborg, like me a lifelong Democrat, wrote a wonderfully subtle putdown of Dukkasis in his fabulous “Chemist in the Whitehouse” book.) I had many run-ins with the anti-nuke front pagers there; my favorite among them was that wonderful woman who liked to represent that she was qualified to rule on the value of Glenn Seaborg’s work at building our nuclear infrastructure because she once had a summer internship at Hanford.
You can’t make that stuff up.
I’m sure that the way I feel about Democrats who are anti-nukes approaches the way that some of the more rational Republicans feel about their creationist wing. There’s simply nothing that can be done with these people, they’re essentially robots who can’t be trained to think independently.
On both sides of the aisle, the contempt for science is a bane to our country and to the planet at large.
On a bright note, I am happy to report that my banning came as a result of telling the truth. Referring to Hansen’s wonderful paper that quantified in a clear and unambiguous way that nuclear energy saves lives, I stated that opposition to nuclear energy is nothing other than murder; I paraphrase but use the exact word that I used: Murder. It happened in your diary.
To his credit, Kos let me write there for many years despite my overt contempt for his, and his front pagers, anti-nuke energy views, contempt about which I made little secret. He is to be applauded for that. But I stopped writing there as I monitored the post-Fukushima increases in carbon dioxide additions and became disgusted and bitter about what anti-nukism has wrought for future generations. I was just too disgusted. Tim’s (Meteor Blades) remarks to me after I experienced Hurricane Sandy played a role in leaving me disgusted with Daily Kos as a whole. Tim’s really an insufferable fool. I really couldn’t write there any more, at least any more than snide comments and jokes on SensibleShoes wonderful Write On! diaries.
But to return to that “murder” word: Kos is an anti-nuke. He has always been an anti-nuke and always will be one. I have characterized that unenlightened class of people with a very strong word, an appropriate word I think, but a strong word that is bound to – and is designed to – offend that class. He owns the website. Whatever his intellectual and moral limits he is perfectly free to ban anyone who offends him and surely I do just that.
My friend Brian Mays, a fine nuclear engineer and scholar, did me a big favor months ago, and downloaded all of my diaries so that their content is saved for what they are worth to me and my family – the personal records and tales I told as asides may be valuable to my sons when I am gone.
So it’s no loss to me personally. I did what I could there, and people sometimes wrote me to tell me that I helped to change their minds – as brusque as I was – and that was gratifying. But mutually, between the Kos staff and myself, we had reached a point of diminishing returns.
I kick around the internet. I also regard Rod as a friend and maybe he’ll let me write here from time to time as he has done here.
Best regards,
NNadir
P.S. You may reach me personally at NNadir.fp@gmail.com
NNadir,
Good to see you posting; I have missed your interesting and fascinating tidbits.
NNadir,
Thanks for the post. A lot of good stuff to think about as usual. Haven’t had to think about orbitals for awhile so good reminder of that subject.
Regards,
That was a refreshing break from the politics and public policy. A very nice read and much food for thought.
nnadir was/is not the only pro-nuke on the DK. I’m one. And there is actually a growing list of them, including Keith Pickering who wrote a wonderful diary on the costs of renewables vs nuclear recently. Only…nnadir is the most…well know both for his science grounded arguments and his take-no-prisoners-war-on-ignorance.
The many strong pro-nuclear community on the DK want’s nnadir to come back!
Rod,
Thanks! Quite interesting. It upgraded my understanding of the periodic table and the differences between lanthanide’s and actinides.
Never knew the potential to use as a catalyst.
Now I also understand why scientists check the level of U in agricultural fields that use phosphate fertilizer.
This even may become quite important due to the Codex Alimentarius Commission new recommended food safety standards (all foods together must deliver <1mSv/year), that Japan already adopted.
Regarding “Carbon Dioxide Capture, Storage and Utilization.”, specifically, the implication that we might continue to burn fossil fuels to make electricity, but capture the carbon, combine it with hydrogen (made with nuclear power), and synthesize transportation fuel:
It is hard for me to see how the economics of this would work, compared to using the nuclear power to make electricity, and the fossil fuel to make transportation fuel, which seems to have much better efficiency. Consider that converting natural gas to methanol has 67% efficiency (according to ANL-339), but conversion to electricity has only 60% efficiency (delivering natural gas as cng has 97% efficiency). Nuclear power to electricity is about 30% efficient, and using electrolyzers results in heat-to-H2 of 21%.
So to me, fuel synthesis only makes sense if the goal is to stop using fossil fuel. That leaves carbon capture from biomass, the air, or water, all of which add cost.
Aviation and small motors such as lawn mowers and chain saws can justify a premium carbon-containing fuel, but driving around town just needs a fuel that’s cheap. Ammonia (NH3) is the cheapest fuel that can be made from nuclear power (or sun, wind, or OTEC). Ammonia is a liquid at under 200 psi, and has an energy density 2x as good as 10,000 psi hydrogen and slightly better than cng, so it doesn’t need an expensive fuel cell to deliver adequate driving range (it works fine in a modified ICE, and delivers higher efficiency than gasoline). Using reverse fuel cells for ammonia synthesis (which take only steam, nitrogen, and electricity as inputs) ammonia could be as cheap as H2 at the plant, and of course much cheaper once it’s delivered to the end user. The reversible ammonia fuel cell has been demonstrated at lab scale using proton conducting ceramics, but requires more development.
The usual complaint about ammonia fuel is the safety issue (ammonia stinks and is toxic when inhaled for prolonged periods). But this is just an engineering issue to be solved. Studies have shown that its transportation by truck and retail storage risks are no worse than gasoline. All that remains is to develop a consumer-friendly fuel dispenser (spill-proof dispensers have been developed for methanol and a fully automated H2 refueling system has been developed, so we can surely make ammonia work). And of course there will be no evaporative emissions, since ammonia is stored under pressure (like cng, but at 20x lower pressure).
Here’s a good intro to ammonia fuel: NH3 – The Other Hydrogen
Here is a presentation on ammonia safety.
The proponents of ammonia fuel do have a point, but it has the same fly in the ointment as hydrogen: it requires a substantial new infrastructure before it can be truly useful, and it’s incompatible with everything we have now. In an era of austerity, large infrastructure projects with long payoffs are not good bets for success.
Our existing “renewable” fuels all start with biomass. Fermentation releases CO2, which is in a fairly pure form and is easily captured and stored. There is work on thermochemical processing of biomass, to convert lignocellulose to sugars and oligomers using little more than hot water at less than 300°C. If we are looking for a relatively small source of liquid fuels with the prospect of carbon capture, biomass can fill the bill. All we need is nuclear heat to drive the process.
@Engineer-Poet
If we replace most coal and natural gas in electricity and industrial heat (plus ship propulsion) we should have plenty of accessible hydrocarbons to last for a very long time in the remaining markets. We should also be pretty close to the point at which natural CO2 sinks can keep the atmosphere in balance. We might need to give nature a little help by planting extra trees, but that’s not such a burden. Most people LIKE trees.
If you look at USA patterns of energy consumption, a lot of gaseous fuels go into residential and commercial buildings. Those have to be replaced too. Just how hard they are to replace I don’t know yet, I have the data but I’ve not had time to crunch it.
There’s an awful lot of biomass produced in the course of human activity, everything from excess cornstalks to rice straw (currently burned to eliminate pests) to municipal tree trimmings, grass clippings and organic garbage. We need better ways of dealing with it. Turning it into fuels and stuffing the related CO2 into e.g. spent oil and gas wells kills multiple birds with one stone.
“If we are looking for a relatively small source of liquid fuels…biomass can fill the bill”
Sure, we in the developed world can probably uses efficiency improvements and battery electric vehicles to cut our liquid fuel use by a factor of two or three. In the mean time, what about the other 6 billion people? Are they to remain in energy poverty, or can they have cars too?
Dave MacKay reports that in Europe, energy crops yield 0.5W/sq.m=0.5MW/sq.km = 2000 sq.km per GWatt. As I recall, when supplemental H2 and heat are added to the biomass, the fuel output per unit area triples, so 670 sq.km per GWatt of fuel. It still sounds like a lot of land to cultivate and cost to avoid the inconvenience of new fuel infrastructure.
Aviation is about 8% of our liquid fuels usage. That seems like a good goal for biofuels to work towards. Must we really clear and farm every square inch of the planet?
It depends if they are smart enough to escape the Malthusian trap. If their response to e.g. the availability of piped-in water is to use the saved time and effort to have more children that they cannot support, they are going to remain in poverty regardless of the resources that Western technology makes available to them. It may be that the sum total of human misery is reduced if such people do not get those technologies.
Of those who stay out of the Malthusian trap (and prevent immigration of people who can’t avoid it themselves), the amount of renewable liquid fuel they can afford depends on the amount of excess biomass they can devote to producing it. The amount they can devote to (non-electric) cars is the excess after essentials are taken care of, such as food and its preparation. Singapore isn’t going to be able to have many cars if it has to depend on its own resources. Fortunately, Singapore can trade goods and services for raw materials, or just rely on subways.
There are more ways to run aircraft than by burning liquids in gas-turbine engines. Beamed power is an under-appreciated technological possibility.
One of the reasons I appreciate nuclear energy is that it displaces both fossil fuels and “biofuels”, removing an argument to convert natural areas to cultivation.
Another interesting application of uranium chemistry is in thermo-chemical hydrogen production. As reported here: ORNL work
“Uranium is used at temperatures well below 700ºC to decompose water (H2O) to produce hydrogen (H2). To accomplish this, steam and other gases are passed through a mixture of uranium oxides and other chemicals at temperatures of 625ºC and above. With these noncorrosive reagents and relatively mild temperatures and pressures, the processing equipment and the construction materials for the required unit operations are commercially available off the shelf. This thermochemical cycle has several advantages over competing processes because the chemical mechanisms proposed require no inventory of volatile hazardous chemicals and have operating temperatures that are compatible with common materials of construction.”
The significance of the 700C temperature is that it is easily produced with any TRISO fueled nuclear reactor: the pebble bed reactor, GT-MHR, and the NGNP HTGR, as well the very promising FHR which combines robust TRISO fuel with molten salt coolant (which improves cost effectiveness by allowing much greater power density), DMSR, and LFTR.
This family of H2 production cycles was passed over in the early nuclear H2 work, since they involve solid intermediate products, which were deemed inconvenient. The formerly preferred cycle, sulfur-iodine (which involves only fluids), requires handling high-pressure sulfuric acid at 900C, which is also quite inconvenient.
Another try here: S06-052: Carbonate Thermochemical Cycle for the Production of Hydrogen. Juan Ferrada, Les Dole, Charles Forsberg, …
@Nathan
I’m not sure how you are attempting to insert the link, but it is leaving off the http: part of the URL. I deleted the duplicates and fixed one of your comments.
You know Nathan, I’m familiar with a lot of hydrogen cycles, but this one is new to me.
You don’t know me, and I’m not bragging but let me tell you that turning me on to a hydrogen cycle I don’t know about is a neat trick at this point in my life.
Thanks much! I’ll check that one out!
Hi NNadir,
Great article, congratulations!
I have posted a summary for it (in Portuguese) on my own pro-nuclear blog. Hope it gets some more people to read it.