Detectable radiation versus dangerous radiation
There is no doubt that ionizing radiation at high enough levels can cause illness or even death. It is, after all, a form of energy that has the ability to do work. Anything that can do work and move physical objects – including tiny physical objects like chromosomes – can also do damage.
However, since ionizing radiation is a part of our natural environment and always has been a part of that environment, there is a point at which the potential damage that it can do falls so low that it cannot be separated from the damage being done by all other influences. Because of the almost miraculous way that living organisms have evolved to be able to repair damage done by the normal external forces that affect them, it is very likely that there is a dose rate at which a certain amount of radiation is actually good because it stimulates those repair mechanisms. This notion of radiation hormesis flies in the face of conventionally accepted radiation protection assumptions, but it is actually quite logical and does not violate any validated scientific theories.
One challenge associated with understanding radiation is the confusing set of units used to answer the very important question of “how much” is there. Though I long ago served for about a year as a radiological controls officer and also reviewed a lot of radiological control logs during my time as an engineer officer, I have had to pay close attention, dig out some old text books and exchange a number of emails with trusted colleagues to bring myself back up to speed on the differences between a RAD and a REM, between a REM and a Sievert, between a Sievert and a Gray, and between a curie and a becquerel. Even among experts with decades of experience in the business of measuring radiation, the correspondence has included questions about decimal places and second (or third) checks to ensure that simple errors do not result in answers that are off by one or several orders of magnitude.
Aside: I have often been called to task by other nuclear communicators for using the phrase “orders of magnitude” because they think that “the general public” does not understand that term. I disagree – it is simple for everyone to understand that the term “order of magnitude” means multiplying (or dividing) by a factor of 10. The difference between a penny and a dime is one order of magnitude. A penny is 11 orders of magnitude smaller than a billion dollars. Anyone who does not understand that terminology should start now.End Aside.
The radiation readings that have been reported from the site of the Fukushima Daiichi nuclear plant are certainly something worth worrying about if you happen to be at the site where they have been measured. There is an accumulation of water in pipes and in the turbine building at unit 2 that has been measured at reading of 1000 millisieverts/hour on the surface.
That water is immediately dangerous to human health – a person who remains in a radiation area with that dose rate for just one hour will begin to get noticeably sick with a total dose of 1 Sv (100 REM). That is four times the one time emergency doses allowed for a radiation worker and 20 times the maximum legal annual dose limit for a radiation worker. It is 400 times more than a somewhat normal annual dose of 250 millirem – 2.5 mSv.
On the other hand, there have also been reports from various measuring stations around the United States of detectable levels of I-131. The levels detected in the US have been in the range of a few tens of picocuries. The prefix “pico” means 12 orders of magnitude smaller than 1, so a picocurie would be 0.000000000001 curies. 100 picocuries is equivalent to 3.7 becquerel, with a becquerel defined as one decay per second.
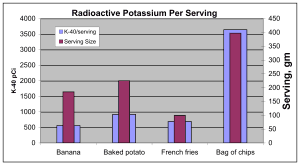
Just to put those tiny numbers into perspective, an acquaintance named Gary L. Troyer, Nuclear Chemist, Computer Scientist, Member: IEEE, ANS, HPS, AAAS, put together a chart that display the average radiation dose rates that come from routine exposures to common food items that contain minute amounts of potassium-40 (K-40) a naturally occurring isotope.
In other words – worry about the effort to contain the highly radioactive water that is on the site at Fukushima Daiichi. Think about the difficulty of the effort that the workers are undertaking to prevent that material from leaking out into the environment before it has a chance to decay away. However, do not worry when your local news reporter tells you that sophisticated measuring devices have determined that there is a detectable level of radioactive isotopes in the air that you are breathing or in the water that you are drinking. Listen carefully to the reported numbers to find out if it is any more than what you would get from eating a banana or a bag of potato chips.

> … challenge associated with understanding
> radiation …
Yes indeed. I don’t think I’ll ever understand it.
The Grey represents the total energy deposited in a given mass of any material, and the Sievert modifies the Grey by way of a weighting factor to account for the absorptivity of a given material (skin vs. gonads, say, for purposes of biological effects) and type of radiation. So far so good?
Where do they account for the amount of radioactive material, or its geometry?
Should I be just as scared of a thimbleful of of that radioactive water as of a gallon?
Should I be equally afraid of a gallon of the stuff in a small sump pit as of a gallon spread all over the floor?
When they talk about radioactive water, they are really talking about the stuff _in_ the water, right? Stupid question: Is distillation not an option for cleaning up this water?
Thanks Rod, and congratulation on the new site.
To Rod, and others who are qualified to comment
Perhaps you can use me as a proxy; like many who will come after me, I was agnostic regarding nuclear . . . prior to Fukushima; and still that way, but now seeking deeper understanding, and trying to intelligently discern science fact from propaganda. And I am cognizant of the fact that I am trying to compress into days an evaluation that for many of you has stretched to decades.
I value what I read here for the honest discussion it seems to attract and the relative lack of thoughtless comments. In particular the gentleman with 20 years experience on Mark I’s (Jim?) has been extremely helpful in helping the determined layman understand some of the finer points, even though I suppose he hasn’t been met with consensus agreement from others here.
I have a question that I can sense is polemic, and I ask it here in sincerity, and only because I respect the honest evaluations that have been given. What about the medical idea that has been forward, on internal radiation dangers, i.e. the supposed dangers of stray isotopes which become presented to us internally, either through breathing or ingestion? Obviously, with a little reading, one begins to sense that the whole debate of long-term safety may rest on affirming or defeating the sensibility of this proposition: it is the pivot of whether the tally from Chernobyl is 1M or else “many orders of magnitude” smaller.
Being new to the literature, I can only see that some proponents of this view have credentials and some proper peer-reviewed studies. Although it is obvious that some, like Dr. Busby, seem to be somewhat scientific pariahs, and the balance of the literature discards their concerns. On the other hand, as a reasonable person I can’t help but notice that the IAEA would have a vested interest in downplaying such concerns, as long as they weren’t directly and unfailingly provable.
In summary, what do you all think of these viewpoints? Again, being new to the literature, the theses behind their claims makes as much sense as low level external radiation being hormetic. If I defend nuclear power for further investment, what do I say regarding this, to those who raise such issues?
Sincerely, Philip
@Philip – thank you for the compliments regarding the reader base here at Atomic Insights. They really do make this site something that is far different from what it would be if there were no comments. The quality of the comments on many aspects of various issues make it worth working hard to start the conversation.
With regard to the hazard of internal doses caused by ingestion of radioactive isotopes, there is a tremendous body of scientific research that should put your mind at ease. One group of people, women who were employed as painters for radium dial watches, have contributed, inadvertently, to our understanding of how very large internal doses can be very damaging. That same group has also helped researchers to understand what happens to people who are exposed at much lower levels. The answer is quite encouraging.
Another group that has helped me to understand just how wrong people like Ralph Nader are when trying to scare people about plutonium is a group of former weapons manufacturing technicians who inadvertently breathed in plutonium dust particles when their protective equipment failed. That group is quite tiny – consisting of a couple of dozen subjects, but the I Pee Pu club was carefully monitored for many decades after their internal exposures occurred.