Comparing A Leak of Tritium That Causes A Flurry to Two Oil Leaks That Are Virtually Ignored
Several of my regular sources of information about the happenings associated with nuclear energy, including John Wheeler’s This Week In Nuclear and Meredith Angwin’s Yes Vermont Yankeehave published stories about the recent flurry of news regarding the discovery of tritium in ground water on the site of the Vermont Yankee Nuclear Power Plant.
I have been following news about Vermont Yankee because the plant’s license is nearing expiration and because the situation in Vermont is unique in that the state actually has a say in whether or not the plant continues operating. Normally nuclear plant license extensions are decisions made by the Nuclear Regulatory Commission. I am also interested because Vermont is ranked near or at the top of the list of states in terms of lowest CO2 emissions per person, largely due to the electricity produced at Vermont Yankee. It would be almost tragic if the well operated plant was closed down prematurely.
I confess – I am interested in numbers and dabble in spreadsheets as part of my daily employment. I understand the definition of an “order of magnitude”, recognize the difference between a “milligram” and a “kilogram”, realize that spending a billion dollars is equivalent to giving 100,000 people a $10,000 check, and know that a curie is defined as the activity level of 1 gram of radium 226 (3.7 x 10^10 decays per second).
In other words, I am hopelessly unrepresentative of the average American. No matter how often someone repeats a story about thousands or even hundreds of thousands of picocuries of tritium, I cannot be frightened into action except when I realize that some people thing that is a big enough deal to cause them to advocate even a momentary interrupt production from an asset that supplies 620 Million watts of clean, affordable, emission free electricity.
Let me try to explain why I am not frightened. Here is what the Environmental Protection Agency says about the hazards of tritium (which is simply an isotope of hydrogen with two neutrons in its nucleus).
Health Effects of Tritium
How does tritium affect people’s health?
As with all ionizing radiation, exposure to tritium increases the risk of developing cancer. However, because it emits very low energy radiation and leaves the body relatively quickly, for a given amount of activity ingested, tritium is one of the least dangerous radionuclides. Since tritium is almost always found as water, it goes directly into soft tissues and organs. The associated dose to these tissues are generally uniform and dependent on the tissues’ water content.
With the knowledge that tritium is worth some minor concern because it is mildly radioactive, I then go through the mental process of determining if “thousands of picocuries” per liter is something to worry about. I know off the top of my head that the drinking water limit in the US is 20,000 picocuries per liter. I also know that a picocurie is just 0.000000000001 curies (1*10^-12) and that one of my heros, Admiral Hyman G. Rickover once volunteered during congressional testimony to drink primary coolant water from a nuclear plant, which has a much higher concentration.

What I was not quite sure of was the mass of one curie of tritium. It turns out to be 0.1 milligram (1 x 10^-4 grams). To the right is a photo of a 385 milligram aspirin; showing a photo of 0.1 milligrams of aspirin would require breaking that tiny tablet into 3,850 pieces.
I did a Google News search on the words “tritium leak Vermont” that produced 113 results, several with associated images of protesters carrying signs advocating an immediate plant shutdown. I read some of the articles and realized that they were stories about a few liters of water in the ground at the site of a nuclear power plant that each contained 0.000000028 curies of tritium. That is just 2.9 trillionths of a gram (2.9 x 10^-12) of tritium. For comparison, every liter of water contains about 111 grams of hydrogen, so the portion of hydrogen that is a tritium isotope is incredibly small – just 1 out of every 2.6 x 10^14 atoms.
The breathless calls for a shutdown of Vermont Yankee due to the quantity of tritium discovered is not just a tempest in a tea pot, it is more like vividly describing a swirl in a water droplet under a microscope and believing it should instill the same level of concern generated by Hurricane Katrina.
I cannot help but compare the tone of press reports that the forces arrayed against the intelligent use of nuclear fission energy are producing with regard to these “leaks” and the tone of press reports about two current oil leaks. If you do a search of news stories for this week, you can find at least two occurrences of large releases of petroleum products. One happened from a Siberian pipeline and is causing some concern by the WWF for its effects on tigers, one happened in Texas near Port Arthur.
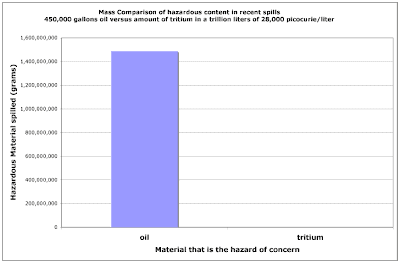
In both cases, the amount of petroleum released onto the ground or into a body of water exceeds 450,000 gallons or about 1.5 million kilogramms (1,500,000,000 grams). Compare that quantity to just 2.9 X 10^-12 grams per liter in the tritiated water found in test wells at the Vermont Yankee nuclear power plant. It is difficult to grasp just how large a magnitude difference that is. Here is a chart comparing the mass of petroleum to the mass of tritium that would be found in a trillion liters of water that contains 28,000 picocuries of tritium per liter. Since the total quantity of tritium is just 2.9 grams in that case, it does not even show up on the chart, it is still in the line thickness of the x-axis.
Here is what the Office of the Attorney General of the State of New York has to say about petroleum health effects:
What are the health effects of exposure to petroleum products?
Health effects from exposure to petroleum products vary depending on the concentration of the substance and the length of time that one is exposed. Breathing petroleum vapors can cause nervous system effects (such as headache, nausea, and dizziness) and respiratory irritation. Very high exposure can cause coma and death. Liquid petroleum products which come in contact with the skin can cause irritation and some can be absorbed through the skin. Chronic exposure to petroleum products may affect the nervous system, blood and kidneys. Gasoline contains small amounts of benzene, a known human carcinogen. Animals exposed to high levels of some petroleum products have developed liver and kidney tumors. Whether specific petroleum products can cause cancer in humans is not known; however, there is evidence that occupationally exposed people in the petroleum refining industry have an increased risk of skin cancer and leukemia.The real contrast in my mind is the fact that though there is some amount of coverage describing the oil spills and the effects, there is no one who is trying to use the spills as the justification for shutting down a valuable production facility or as a public relations weapon to beat up the management of the responsible companies. Certainly there is no one who is gleefully pointing to these oil leaks as a reason for attempting to shutdown the entire oil industry, but a blog post about tritium leaks starts with Comment: No to Nukes, they are dangerous and deadly! When oil leaks, people get concerned, but they take it in stride, minimize the harm and clean it up. No one would be willing to forgo all of the benefits that they obtain by burning oil merely because it is a liquid that sometimes leaks, even when it leaks in multi-tonne quantities.
Why is it that when tritium leaks, it is used to cause fear, uncertainty and doubt about the value of producing emission free, affordable electricity or heat using nuclear energy, even when the amount of material in question is measured in micrograms?
PS – Please feel free to check my numbers. I am perfectly capable of slipping a digit here and there.

I notice the choice of units in the press leads to large numbers, to frighten people. The press reports “20,000 picocuries”, not “20 nanocuries” nor “0.2 microcuries”.
“For comparison, every liter of water contains about 111 grams of hydrogen, so the portion of hydrogen that is a tritium isotope is incredibly small – just 1 out of every 2.6 x 10^-14 atoms.”
Should this be “2.6 * 10^14”? I can’t see how you can have one tritium atom for every 0.0000… atoms. By your wording, this should be a large number, not a small one, right? It is early, so maybe I’m just misunderstanding you.
Yes there is an misplaced negative sign. It should be 1 out of every 2.6 x 10^14 atoms.
Ah, why keep to the facts when emotion gets you further?
Look at what happened at Brookhaven National Lab. The M&O contractor lost their contract over their tritium “problem.” A new template for anti-nuclear activism.
There is a great deal of just that in the antinuclear movement. There is a percentage that are just out to be part of some movement that is doing something, anything, and they are not really interested in thinking too much about the issues, as much as they are getting high on the act of protesting itself. It is a social activity for them, and a chance to meet and be with other people in an invigorating environment. This is particularly true in the protest side of the movement, the ones that go out to picket a NPP, it’s a chance to get together like some sort of a club field trip.
I have questioned members of such demonstrations on more than one occasion, and I still am appalled at how little some individuals knew about the issues they where nominally protesting – but boy where they having fun.
This reminds me of another incredibly skewed application of justice in regards to a fine levied against Exxon for killing 85 protected birds:
http://dallas.bizjournals.com/dallas/stories/2009/08/10/daily46.html
So why is it that Exxon is fined for bird killing but wind farms are not? Not taking any sides, just pointing out that the environmental damage done by wind farms to birds far exceeds this one incident.
These types of issues are where my cynicism of the anti-nuclear industry really kicks in.
They get their faithful wound up about a tritium leak where the concern is the supposed harm to HUMANS due to potential for the water table to be contaminated. While 2000 miles away ANIMALS are being harmed due to the petroleum spill and they are not outraged. It seems like every time where tritium is used as a bludgeoning tool against nuclear power, they only mention the HUGE, INCREDIBLY SIGNIFICANT damage that MIGHT occur to humans, never mentioning what might happen to the animals who are more likely to drink straight from the water table. Is the animal life around the Vermont Yankee
Time to coin a new term: “Big Enviro”? Green is the color of money.
I don’t think it makes sense to use mass (“trillionths of gram”) as a common reference point. Toxicities vary by many orders of magnitude.
CEDE of tritium ingestion is 0.64 Sv/Ci. 20 nCi/L means ingesting 1 L commits a dose of 13 nSv, or 0.2% of daily background dose (though actually it’s spread out over 10 days). Eating a banana is 100 nSv.
The regulatory limit seems extreme. If one were to drink nothing but 20 nCi/L tritiated water, 2 L/day (the intent behind “drinking water” standards — something you can drink indefinitely), the accumulated dose would be 9.3 uSv/year – 0.4% of background. 90 bananas/year, or one two hour airplane flight per year.
Cf. CNSC:
http://www.cnsc-ccsn.gc.ca/eng/mediacentre/updates/tritium_drinking_water_aug_2009.cfm
“Safe” levels vary wildly — almost five orders of magnitude in tritium standards. The US is in the logarithmic middle. The simplest explanation is this is all witch medicine; there is no empirical justification for any of these numbers, so they pull them out of thin air.
The acute LD50, incidentally – assuming a radiation LD50 of 4 Sv (from wikipedia) – would be 300,000 m^3 of this “contaminated” water (300 million liters or 75 million gallons, or 150 olympic swimming pools).
Of course it’s impossible there’s that much contaminated water in total. Say there is on the order of 100 m^3 groundwater involved (generously large). Then there is 2 mCi of tritium present, which would be trivial in any other industry but nuclear power. From various sources: tritium-illuminated wristwatches contain roughly 2 mCi tritium, gunsights contain 12 mCi, and exit signs 25,000 mCi (25 Curies).
Sorry, I wrote the previous comment. For some reason the comment system changed my name to “Guest”…
Benefits of Low Dose Radiation
I have researched the literature for evidence of benefits from low dose radiation. John Tjostem
A process known as radiation hormesis mediates its beneficial effect on health. Investigators have found that small doses of radiation have a stimulating and protective effect on cellular function. It stimulates immune defenses, prevents oxidative DNA damage, induces DNA repair enzymes and suppresses cancer. Government authorities and regulators
The problem is, and always has been, the media that sensationalizes these sorts of things, rather than examining them in a mature fashion and providing the necessary context for the reader/listener/viewer to come to an informed conclusion. Informed conclusions don’t attract an audience, unfortunately; drama, even if it is manufactured, does.
(RADIATION! IN OUR WATERS! WON’T SOMEBODY PLEASE THINK OF THE CHILDREN????!!!!!!1!111!!1!11)
Dave – that may have been true at a time when only large, well capitalized publications could reach a widespread audience. Even though the enterprises had substantial start-up capital, they had to develop an income generating model to provide sustaining revenue; no amount of capital can last forever when the burn rate includes support for printing presses, massive purchases of paper, trucks, delivery personnel, and rack space or broadcast television stations, film crews, pretty faces, and networks of transmitters.
None of the traditional media outlets could survive based on charging their customers, though they do get some amount of income from subscriptions. Instead, the model that developed was a commercial one with the word “commercial” meaning advertisement, not commerce. If you make money by selling ads, you need to reach a lot of eyeballs, so sensationalism becomes part of the mix. If you want to keep your advertisers happy, you write stories that do that by promoting the products or the industry AND by writing stories that knock down the competitors.
Here is a hobby for you. Subscribe to a raft of magazines and pay attention to just how many times you find full page ads from Shell, Chevron, ExxonMobil, Valero, Peabody Coal, Chesapeake Energy, etc. Think about the sponsorships you have seen on the television over the years. Then try to find even a tiny bit of advertising from a company focused on selling nuclear technology.
I am a weak enough person to recognize the siren song of money.
BTW – the Internet model of publishing offers a way around this kind of media. It is widely accessible, yet the costs of production can be managed on a completely different scale – sort of like the difference in magnitude between tritium and oil contamination.
Don’t tell anyone this but I caused a tritium spill that was far greater than this in the parking lot of a Blockbuster Video about a year ago. I tossed my friend my keys to get something out of the car. He didn’t catch them. They hit the ground and the little glass cylinder containing many milicuries of tritium broke. I was bummed. I had to get a new one.
Steve: Interesting story. I am a deeply cynical person who thinks about stories like that, and also thinks about just how much havoc an anti-nuclear group could cause by breaking a key chain decoration and spreading the resulting fractions of a microgram of tritium onto the ground of a nuclear plant. All they would need would be bribing a single roving guard to do the deed. I hesitate to write this for fear of giving them ideas, but then again, if people knew just how easy it would be to insert measurable quantities of tritium anywhere, they might get lose their initial fear. In this case, I guess I am not too concerned about spreading ideas since the act would not cause anyone any physical harm – even if the “attackers” figured out how to use millions of times more tritium. The stuff is simply not very dangerous, but it sure is easy to measure in exceedingly tiny amounts.
Steve – not “many millicuries” but “millions of picocuries”. Get it right or people won’t be scared enough :-/ .
Rod – with the blessing of the NRC, someone should do exactly that, deliberately spill an obtainable tritium source, and see how traceable it is (and how distinct it is from other causes). Perhaps a research lab already has.
Joffan – wouldn’t “millions of picocuries” still be several microcuries? You have to get to a billion picocuries before you make it to a single millicurie.
I have a radiolumiscent key fob that originally contained ~1 curie of tritium. If I break it I can make 50 million litres of water with a concentration of 20 000 pCi tritium per litre.
Rod, now you really made me scared with that low level of tritium, hence I wil stop drinking water. Instead, I will drink beer made with hops grown in Chernobyl. Knowing that low level radiation is beneficial to my health it is the only prudent action one can take to get a little radioativity one needs.
My recollection is that it was Bernard Cohen who offered to drink reactor coolant water at a congressional hearing but can’t find a decent reference to confirm either he or Rickover was willing to do it. I am doubtful Rickover said it because he was actually fairly skeptical of commercial nuclear power and testified as much at congressional hearings. He was only in favor of nuclear reactors in submarines because it was such a game changing technology for that application.
The kind of comparisons you make are reasonably logical to people like us who work in the industry. However, to my mind, a key problem has always been education of the public. Until high school kids are taught about radiation and given a practical understanding of nuclear, this kind of emotional sensationalism will continue indefinitely.