Tritium – aka radioactive hydrogen – from reactors is not a threat to human health
Tritium, also known as radioactive hydrogen, is an isotope that releases an 18 Kev beta particle. The isotopic half life is about 12 years.
Among other possible production mechanisms, it is produced in low quantities and concentrations in any reactor where water is exposed to a neutron flux. The production rate is higher in heavy water reactors because the deuterium (H-2) that is a major constituent of heavy water only needs to absorb one more neutron to turn it into tritium (H-3). Its absorption cross-section is not very large, but there is no loss term in the build up equation.
Over the years, there has been a great deal of ink spilled in articles, reports, and legal proceedings regarding the potential impacts of releasing tritium to the environment. The stimulus for this post is an article by David Biello dated February 14, 2014 titled Is Radioactive Hydrogen in Drinking Water a Cancer Threat? .
Aside: Yes, I know. That article is more than a year old. I didn’t look at the date until I began writing this post. Apparently SciAm is doing what I occasionally do here, which is to repurpose existing content. Here is a quote of the note at the top of the article “This article is from the In-Depth Report 4 Years after Fukushima.” That in-depth report was published this year, not last. End Aside.
You might want to read the original article to obtain more context for the comment that I’ve reprinted below.
It is disappointing to read an article in Scientific American that provides such a scientifically inaccurate description of the way that the beta particles from tritium (free moving electrons that are only slightly accelerated compared to those produced in a normal electrical circuit) react with matter.
Those electrons do not “slam into DNA, a ribosome or some other biologically important molecule.” They interact with the electrons in all of the matter that they pass through, including air and water. Even in air, beta particles from tritium decay travel a MAXIMUM distance of 6 mm.
In more dense material, like biological matter, they travel a substantially shorter distance.
Living cells are composed primarily of water. DNA and other biologically important molecules are a small portion of the total mass. Most of the energy in the slow moving beta from tritium gets shared with the electron clouds of other molecules through inelastic collisions and the Bremsstrahlung effect, which turns the kinetic energy of the beta emission into electromagnetic radiation. That is the same kind of radiation that surrounds us in our modern, electrified lives.
The other important feature of tritium is that it is an isotope of hydrogen, one of the most common elements in our environment. When you mix tritium with water, the isotope inherently mixes with all other hydrogen isotopes, resulting in an irreversible dilution.
Anyone with any reasonable understanding of physics will know that a mixture of isotopes of the same element is nearly impossible to separate into the constituent isotopes without the imposition of a great deal of energy and some pretty fancy technology.
No natural biological process has any means of concentrating tritium once is is released into an environment full of other hydrogen isotopes.
It is absurdly amusing to read the following, knowing how much money has been wasted over the years in futile attempts to remove tritium from water to “protect” people against a non-existent threat.
“You need huge study populations to have any chance of seeing anything,” Kocher notes, and that money is simply unavailable. “There is no compelling need to spend the money required to do this.”
If there is no compelling need to spend money to find out that tritium at the concentrations that can possibly be accessible to humans is not hazardous, then there is no compelling need to spend money to attempt to capture it or separate it from water.
Let dilution be the solution to this particular phantom worry.
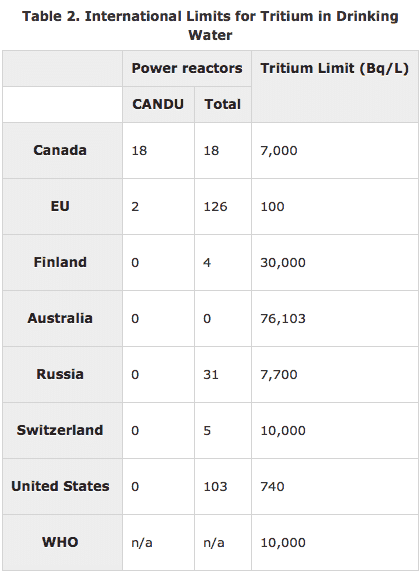
Of course, the money that has been spent on various solutions to the non-problem of tritium has been “revenue” for a large number of parties who are interested in maintaining or tightening the existing regulations. Instead of looking to variations in state rules, I wish the author had turned to our northern neighbors to find out that their drinking water limit is 10 times higher than ours or to Australia to find out theirs is 100 times higher.
In neither case is drinking water full of tritium at the limit dangerous to human beings.
Rod Adams
Publisher, Atomic Insights
There is a large, expensive, and worrisome tank farm full of tritiated water on the site of what used to be the Fukushima Daiichi Nuclear Power station. That tank farm is complicating the process of cleaning up the site. The most logical, lowest cost solution is to simply empty the tanks into the adjacent sea and to recycle the materials used to construct the tanks into something more useful.



These things are relative. And a tritium beta particle is actually only about 6 kev, about a fifth that found in a normal CRT computer monitor. Most normal people might think that rather high. A normal radio or x-ray or radar operator might not. Either way, an 6 kev beta particle is on the very low end of nuclear decay energies, and probably the next best thing to being completely innocuous.
Yes, in a CRT tube electrons are faster than those emitted from Tritium. However, in a normal electric circuit, electrons only move at snails pace, whereas 6keV electrons move at an impressive 7.6% of the speed of light.
So the beta emission from tritium is undoubtedly ionising radiation, what makes it relatively harmless is
1) the low dose factor due to low energy and low biological half life,
2) The fact that ingestion is the only exposure pathway
3) The lack of bioaccumulation and the fast dilution in the environment.
Coming back to the CRT example, typical beam currents of 1-2 mA would correspond to 6 to 12 PBq of electrons hitting the screen.
There’s a very interesting website called The Hiroshima Syndrome, with a lot of entries fun to read and informative. It has an entry on tritium as well:
http://www.hiroshimasyndrome.com/background-information-on-tritium.html
Pretty shocking.
From an MSDS, I got a figure of 1.73*10e-11 Sv/Bq for ingesting tritium in water.
Imagine drinking the 630,000 Bq/liter that all the journalists and bloggers are writing about. Imagine drinking this waste water as your primary water source, 2 liters a day, all year.
That’s 630e3*2*365 = 460,000,000 Bq/year. Sounds like a lot huh? Well, 460e6*1.73e-11 = 0.008 Sv/year. 8 mSv/year.
That’s as much as living in Denver. Imagine that, living in Denver is like, drinking the evil contaminated waste water in the evil tanks from the evil 2011 fukushima apocalypse!
Just for comparison, if you drank nothing but water tritiated to the Finland limit, at a standard rate of 3.7 L/day, you would get 1 mSv of additional radiation per year.
I agree with Rod.
Let’s leave aside the more valid and reasonable argument of insignificant increase of the total radiation background that is associated with tritium. Even on a philosophical level, the issue of tritium cannot be dealt with the same way as with cesium-137, which is present in the environment almost exclusively due to human activity, because tritium has and always had a (radioanalytically) significant constant presence in nature.
There is a nice overview of the topic of tritium in the environment made by the French institute for radioprotection (IRSN):
http://www.irsn.fr/EN/Research/publications-documentation/radionuclides-sheets/environment/Documents/Tritium_UK.pdf
Let me sum up the most interesting points/numbers:
Tritium is being constantly introduced to the environment due to comic radiation production (in one year it is around 0.15-0.20 kg, equiv. to 50 – 70 x 10^15 Bq = 50-70 PBq). Since it is always present in water, its mobility in the environment is of course very high. Given the 12 year half-life of H-3, the saturation amount is around 2.5-3.5 kg (800 – 1200 PBq) total.
By neutron capture at deuterium in its moderator/coolant, a 1 GWe heavy water reactor produces approximately 0.002 kg per year, light water reactors produce approx. 100x less – most of it due to a different mechanism: (n,alpha) reactions during boron-10 capture. The reprocessing facilities at La Hague and at Sellafield open up the inventory of the used nuclear fuel, which contains also ternary fission tritium – this is the source that is being currently discussed at Fukushima. This tritium is being released into the adjacent sea/ocean.
Every year, approximately 1600 metric tons of used nuclear fuel is reprocessed at La Hague, releasing around 30 grams/y (0.03 kg/y) of tritium, while Sellafield releases less – approximately 8 grams/y. The saturation amount from these releases 0.038 kg/y (13 PBq/y) in the environment is approximately 0.66 kg (220 PBq), four times lower than the saturation amount from natural cosmic ray production in the atmosphere.
All these amount fade in comparison to the tritium releases during atmospheric nuclear weapon testing, when an estimated 650 kg of tritium was released to the environment. Since producing tritium and maintaining a sufficient amount for the use in hydrogen bombs is hard, I guess that most of this amount came from in-explosion tritium breeding by (n,alpha) reactions on lithium-6 deuteride (please, correct me, if my guess is wrong).
Due to its short half-life, most of this “bomb” tritium has already decayed, but there is still around 30 kg extra tritium in the environment. Compared to this amount, the saturation amount from current nuclear facilities is quite insignificant and the total amount of tritium in the environment will be dropping for at least 2-3 more decades (..well, while hoping that no more atmospheric hydrogen bomb explosions take place on Earth).
PS: A similar issue concerns the environmental presence krypton-85 (half-life of 10.7y), which is also routinely released from nuclear power facilities (especially from reprocessing plants). In contrast with tritium, the estimate of production of Kr-85 by cosmic radiation is quite low (0.05PBq/y), approximately 10 000 times smaller than current artificial releases (500 PBq/y), so the arguments presented above for tritium won’t hold in this case. On the other hand, due to the rapid dilution into atmosphere and very limited retention in the human body, the final radiation impact from Kr-85 is likely even less significant than for tritium.
For a study how Kr-85 mixes in the atmosphere, see: http://www.mpimet.mpg.de/fileadmin/publikationen/Reports/WEB_BzE_82.pdf
Excellent summary. The total tritium inventory from ternary fission in the F1 1-3 cores should be no more than 1PBq.
For comparison, about 10000PBq of the 230000PBq bomb tritium is still in the environment. About 1.6 PBq of this decays every day.
This means, that even if all the Fukushima tritium was released in a single day, the total global tritium inventory in the environment would still decline on that day.
Scientific American started going down hill when they started taking full page anti-nuclear advertisements from the UCS back in the late 70s or early 80s. They seem to be fully captured at this point.
So, with American Atomics in Tucson, was the tritium contamination real, and, if real, was it really a health hazard?
@Alison Maynard
American Atomics of Tuscon dealt with concentrated tritium used in a variety of lighting products. It was not the kind of isotopicly diluted tritium that is produced by the operation of a power reactor.
There were somewhere around 300,000 curies released. THAT is a problem and a potential health risk.
Update – Based on additional calculations provided here, I retract my statement. There is no health risk from even a release of 300,000 curies of tritium. Under current law, the enterprise that released it would be forced out of business and a lot of money might be expended in the clean up, but there is no risk of harm to people.
Not really. 300kci is about 30 grams. Not likely to harm a single soul unless deliberately ingested. Then it may kill 1 person.
Eating 30 grams of aspirin may also kill you.
There is not a single documented case of tritium death
Reportedly, NCRP Report No. 62 about Tritium in the Environment documents several case of tritium death by ARS, resulting from bone marrow cells destruction caused by exposures over several thousand rem, as is described in the following document ( http://oehha.ca.gov/water/phg/pdf/draft_tritium.pdf
). This report is still behind a paywall, http://www.ncrppublications.org/Reports/062
However there’s also a well documented case of a person incorporating about 35 GBq from a 1,7 TBq release, http://house.rbc.kyoto-u.ac.jp/db/MSSFiles/THO-Accident.htm with no health consequence at all even 11 years later : http://www.ncbi.nlm.nih.gov/pubmed/9652812
Well with 4 links, my comment had no chance to go though the moderation, I hope Rod restores it.
So apparently the NCRP has documented some deaths caused by very, very, very high level of exposures. However exposure to just very high level is documented to cause no harm.
Rod, I did a little calculation that may interest you.
300,000 Ci is 110,000,000,000,000 Bq.
At the dose conversion factor of 1.73*10e-11 Sv/Bq, this makes for 635 Sv if 100% of this material is ingested.
Based on the late Cohen’s book,
http://www.phyast.pitt.edu/~blc/book/chapter11.html
and assuming very pessimistically, 100% of the tritium to find a river, and using Cohen’s probability of 0.0001 for a river water molecule to get into a human, we expect this 300,000 curies to result in a collective dose of 0.0645 Sv.
Based on the pessimistic LNT plus pessimistic assumption of all of the tritium reaching mobile water, we expect around 0.01 death.
Based on actual experiments with mice, we expect 0 deaths with 100% certainty.
This amount of tritium activity might be found in about 12-15 tritium exit signs. However, as Rod points out, radioactive hydrogen comes in as many forms as hydrogen; and that is why the limits were written for drinking water. There’s alot of Curies in those signs, but finding a realistic non-occupational pathway by which they would enter the body is science fiction. If you throw them all in the dump, as did Walmart a few years ago, the pathway is still through drinking water.
Quick note on Bremsstrahlung: Only 0.01% of energy is lost by this process for 10keV electrons in human tissue. See http://physics.nist.gov/PhysRefData/Star/Text/intro.html.
Light atoms are not very good as an x-ray source!
To see full scale Tritium silliness in action, look no further than Germany where the federal radiation protection agency (BfS) under the leadership of Wolfram Koenig prides itself for reducing the tritium concentration in salt water dumped into a salt mine from 200Bq/l to below 40Bq/l. Indeel they now achieve 3-4Bq/l. And they still measure it every month, just in case.
http://www.asse.bund.de/EN/3_WhatHappens/OperationOfMine/SalineSolutionsManagement/_node.html.
Btw Wolfram Koenig was appointed in 1998 by green environment Minister Trittin. He has no qualifications apart from being rabidly anti-nuclear. In fact he is landscape gardener with a diploma for urban planning. In spite of several changes of government, he is still in post because no-one has the courage to confront the anti-nuclear lobby.
Imagine a die-hard Jehovah’s Witness heading the regulator of the blood transfusion service, who would then blatently abuse his position to make transfusions and similar devil’s work not safer, but impossible.
This relevant. It came out this month.
http://www.nuclearsafety.gc.ca/eng/resources/health/tritium/tritium-sewage-sludge.cfm
As you probably know Canada allows higher levels of Tritium than the US.
Would be nice if they divulged the actual analytical results in Bq/l and the actual dose estimates. “Well below” could mean anything from pico-Sievert to 0.1 mSv.
Since sewage workers presumably don’t eat the stuff, the only exposure pathway would be by inhalation. It seems a bit worrying that this dose is high enough to warrant a calculation.
If you can get the report, could you share the numbers here?